Today's article is a guest post by my friend Ben Spielberg, a scientist and operator—but not a physician—who lives in LA. His prior columns include hits like:
and…
Today, he shares his reflections on a non-trivial question:
“What does brain stimulation with transcranial stimulation actually do?”
I'm a TMS doctor, and I'm not sure I have an easy answer for that question either. I do a little editing of Ben’s piece, mostly to cut down on less interesting hyperlinks.
If you're interested in treatment with transcranial magnetic stimulation for your depression, OCD, anxious depression, and other indications off-label, feel free to use the links below. Ben’s practice is in LA:
My practice locations are currently in New York and South Carolina:
And now…handing it off to Ben:
I really love Transcranial Magnetic Stimulation. I first heard about TMS a number of times in graduate school, but it was in a different context that many readers of this newsletter might be unfamiliar with; in neuroscience, TMS is sometimes used as a rudimentary way to image the brain. It can be used to create “temporary lesions” to help map out which areas might be partially responsible for a specific behavior or perception. It can also be used to help monitor the health of different neural pathways, such as the corticospinal tract (which is used today to calibrate the device to each individual, or to find their so-called “motor thresholds” I found this concept fascinating; how can a device that emits magnetic pulses similar to that of an MRI machine affect the brain so impactfully, yet temporarily? But it wasn’t until I worked at a now-defunct treatment-resistant depression clinic abutting Washington Square Park that I saw just how effective it could be as a treatment.
My reasons for becoming a believer are probably similar to many readers of this newsletter: I helped to treat a patient who had trialed dozens of oral antidepressants, and who had cycled through a seemingly endless series of mental health providers, get better before my very eyes. He went from complete and utter melancholy to smiling when he walked through the door, laughing at my jokes as I changed coil positioning during his sessions. He then went out and started living his life again. No longer were his days relegated to his bed. It was like magic–only it wasn’t magic, it was science.
As I began to delve deeper into the world of TMS, and eventually was accepted into a PhD program at Maastricht University while running a busy TMS clinic in Los Angeles, I found it curious that good explanations of the mechanisms of TMS are surprisingly difficult to find. Perhaps it has been explained to you, the reader, that TMS increases neuroplasticity! Or perhaps it increases the downstream signaling mechanisms that increase neuroplasticity! Or maybe it has been explained to you that TMS increases BDNF and decreases inflammatory cytokines? This is also not necessarily incorrect. The problem is that every other treatment for depression also does these things. Psychedelics, SSRIs, psychotherapy - you name it, and it decreases BDNF, increases neuroplasticity, and can even change your gut microbiome. What gives TMS, and what makes it different?
A Quick Neurotransmitter Analysis
Medications for depression are sold to psychiatric professionals based on how they modulate neurotransmitters. Common antidepressants may increase the reuptake of serotonin in the synapse in specific areas of the brain. Or maybe they do the same for norepinephrine. Or, perhaps, they work on particular subunits of monoamine neurotransmitter receptors. However, most research exploring how TMS actually changes neurotransmitter concentration in specific brain regions is rather unremarkable, illuminating slight changes but too inconsistent to derive meaning, or seems more helpful (but practically unrealistic) as potential biomarkers for response than as outcomes after treatment.
A Convergence of Science and Theory
When treating depression with TMS, we typically begin by placing the TMS coil over the left dorsolateral prefrontal cortex (LDLPFC). In the early 1990s, when TMS was an emerging research interest in the treatment of mood disorders, the LDLPFC location was chosen partly as a result of the convergence of clinical neuroscience and psychological theory.
The LDLPFC
In the late 1980s and early 1990s, fMRI machines were not as readily available as they are now. As such, the functional imaging of choice was typically PET scans (and to a lesser extent SPECT scans, which had a brief moment of popularity before being relegated to a few specialized practices to this day, like Amen Clinics1). PET scans can roughly show us where parts of the brain are more or less active, based on markers such as oxygenation, glucose metabolism, and blood flow. At the time, there was a growing and robust evidence base that patients with depression have hypoactivity in their LDLPFC compared to those without. By the way, there weren’t any differences in unipolar or bipolar depression in these studies–both showed a relative lack of activity in the LDLPFC.
The Valence Theory of Emotion
The Valence Theory of Emotion posits that the left DLPFC and right DLPFC have an interesting push-pull relationship, such that the LDLPFC manages positive emotions and goal-directed activity, while the RDLPFC manages threat direction, negative emotions, and withdrawal behaviors. When I first learned TMS, this was still the prevalent theory as to how it all worked: a hypoactive LDLPFC caused depressive symptoms, and a hyperactive RDLPFC caused anxiety symptoms. As such, the goal of TMS is relatively simple: “strike a balance between the two hemispheres.” While this theory appears to have been largely overlooked in recent literature, there is also some evidence from other imaging studies (e.g., EEG studies that reveal alpha wave asymmetry between the right and left prefrontal cortices in patients with depression). In my experience, I do notice that inhibitory protocols at the RDLPFC do tend to reduce anxiety, and excitatory protocols at the LDLPFC do tend to work better for depressive-specific symptoms, but these assumptions are not especially helpful when treating patients at an individual level.
Putting them together
So, we have good evidence that TMS for depression could be applied to LDLPFC because that’s where there are issues of “hypoactivity,” and they even correlate with symptoms of depression, and when we treat patients there, their MADRS, BDI, and PHQ9 scores all seem to improve. However, we don’t actually see improvements when we look at the brain. Despite robust and powerful clinical differences, we see little to no change in functional imaging before and after a series of TMS in the LDLPFC. Note that we do see immediate differences in cortical excitability; it’s just that long-term changes in LDLPFC metabolism are nowhere to be found. Even PET-targeted TMS, where the coil is specifically placed over the most hypoactive part of the prefrontal cortex, fails to elicit a positive response.
Going Deeper
A compelling reason as to why the benefits of TMS don’t show up in the same place as where we see deficiency is that we are merely tapping into a node on a specific pathway. Over the past two decades or so, the use of fMRI has illuminated that one specific part of the brain is implicated in depression: the subgenual anterior cingulate cortex (sgACC). Anatomically, the cingulate is a part of the cortex nestled underneath another part of the cortex, which makes it
harder to reach than more superficial areas of the brain. The subgenual portion of the cingulate is at the very bottom of the cingulate, which makes it virtually impossible to reach with the TMS machines we have available today. Functionally, the sgACC seems to be highly related to depression. If you imagine that the amygdala lights up in an fMRI scanner when a stimulus evokes fear, you can imagine that the sgACC lights up in an fMRI scanner when a stimulus evokes sadness. In fact, the sgACC has been reported to be so highly selective to sadness that it dwarfs any other emotion in response. Not only that, but one study found that the length of the depressive episode is highly correlated with thalamic sgACC functional connectivity.
The sgACC is functionally connected to both the amygdala and the prefrontal cortex. It helps to somewhat directly regulate amygdala activity, but it also holds a bidirectional relationship with the prefrontal cortex. This relationship is thought to be that of anticorrelation 3 –meaning that if the prefrontal cortex is activated, the sgACC is inhibited and vice versa. In depression, if we know that the LDLPFC is hypoactive, it makes sense that stimulating the LDLPFC leads to increased activity, which allows it to sufficiently inhibit the sgACC. This seems to be partly how the SAINT/SNT protocol, which boasts a 79% remission rate after 5 days, was born (and I highly encourage anyone to read the supplementary material to the landmark 2020 study, which elegantly explains this thought process in more detail). According to the publicly available papers, the SAINT/SNT protocol finds a site at the LDLPFC that is most highly anticorrelated with the sgACC, and uses that site to place the coil in an accelerated paradigm. However, it should also be noted that there is emerging evidence that finding the site at LDLPFC with the highest correlation (not anticorrelation) to the sgACC provides comparable clinical results to SAINT/SNT, which has implications I don’t even want to think about right now.
One study has shown that in individuals who respond to TMS, sgACC hyperactivity is reduced compared to baseline activity, and this reduction correlates with clinical responses. But is our core pathway–representing functional connectivity between sgACC to the LDLPFC–meaningfully modulated after a series of TMS? Again, we observe that sgACC functional connectivity is sometimes selectively modulated in responders, that sgACC-LDLPFC functional connectivity increases after TMS, and that at other times, the LDLPFC-sgACC pathway doesn’t change significantly after TMS (even though remission rates do). It seems that some structural changes in this pathway are related to clinical response. By the way, we also know that antidepressant medication can modulate this pathway, such that sgACC-LDLPFC connectivity is increased after successful treatment, which leads one to believe that this might be a non-specific marker of depression as opposed to the actual target.
A Glossary on Resting State Functional Connectivity
When you take a Psychology 101 class, you might learn that specific parts of the brain do specific things. Unfortunately, this list is shorter than one might expect. Most human functions are complicated and involve multiple parts of the brain working at the same time. Sometimes, there are large tracts that easily show us how these parts of the brain connect to each other, which might be referred to as structural pathways. You can see these tracts in beautifully rendered scans using techniques such as Diffusion Tensor Imaging. When we speak about functional connectivity, we refer to areas of the brain that seem to be working at the same time (remember the adage “neurons that fire together, wire together?”), which tells us that they are a functional pathway, as opposed to a clear structural pathway. “Resting state” functional connectivity means that we are imaging the brain while the person is not doing anything and is essentially daydreaming, which will trigger activity from the Default Mode Network (DMN). The Triple Network Model and TMS
There are 3 functional networks that are really important in psychiatry: the default mode network, which is active at rest and stands out when daydreaming or mind-wandering, the salience network which tells our brain to pay attention to things in the outside world, and the central executive network which kicks in when we’re solving problems or focusing on tasks. The default mode network should be anticorrelated 4 with the central executive network, meaning that when one is “on,” the other is “off” and vice versa. In depression, the default mode network is on too much when it shouldn’t be, leading people to focus inwards instead of outwards. To illustrate this point, my TMS clinic in Santa Monica has a hallway with colorful pictures of brains from different animals, and the TMS room is at the end of the hallway. One time, as I led a patient down the hallway who was ⅔ of her way through a TMS course, she asked if the art was new.
The art was not new. In fact, it had been up the whole time–her brain was “stuck” in a DMN-dominated state, and she was only just emerging to recognize new inputs in the outside world.
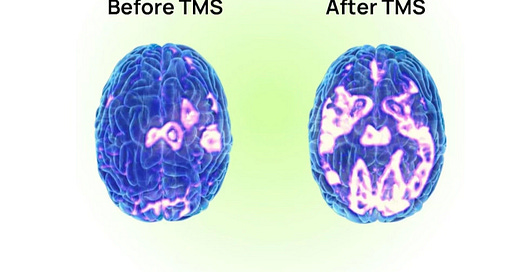
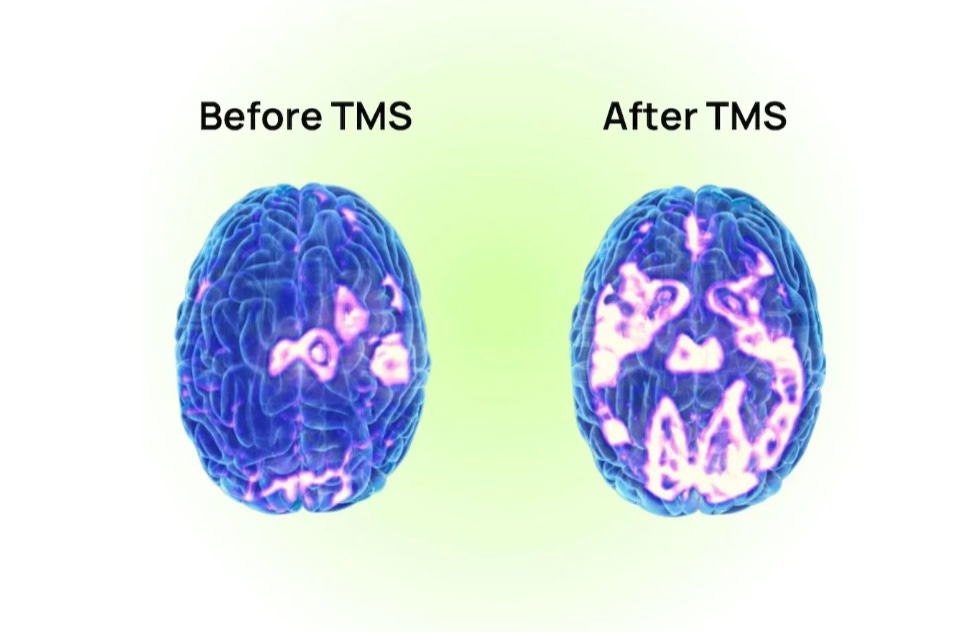
Conveniently, the LDLPFC can be considered a hub of the central executive network. So when we do TMS, we strengthen the central executive network, which can then inhibit the DMN, and thus theoretically reduce symptoms of depression. Indeed, we have some data that a course of TMS will reduce hyperactivity within the Default Mode Network, and that the TMS can reduce connectivity between the sgACC and the DMN. And when we examine TMS responders versus non-responders, we convincingly find that non-responders were more likely to have received stimulation at LDLPFC nodes functionally connected to the DMN, rather than to SN/CEN nodes. But, what if there is another way to think about what TMS does?
Parcels, Anomalies, Connectomes, and Matrices
While we mainly think of protocols in terms of cortical excitatory or inhibitory response, it seems that TMS might more powerfully break up local hyperconnectivity or hypoconnectivity, depending on the protocol used. So, for those with access to an fMRI machine and data from the Human Connectome Project, you can divide the brain up into hundreds of different parcels with varying degrees of interconnectedness, and then create a matrix that identifies aberrant Hyper-connectivity and/or hypo-connectivity between different functional networks. In lay terms, it can tell you when a network is too tightly bound and rigid, or too loosely held and fragmented.
One study in Australia employed this approach, applying iTBS when these networks were hyperconnected and cTBS when they were hypoconnected. The results were compelling, with about 62% of participants achieving remission after 5 sessions per day for 5 days. The same group repeated this targeting approach on a population with primary anxiety (although many of the participants had comorbid mood disorders) 6 as opposed to solely MDD, with compelling remission rates that persisted for over a month post-treatment. Another small study employed a similar parcel-guided approach for placement in participants who failed regular TMS, but didn’t adjust the actual TMS protocol in cases of aberrant hyperconnectivity. Regardless, remission rates hovered at about 50%, and connectivity between the DMN and LDLPFC increased following TMS.
Conclusion

So, what exactly does TMS do? We’ve gone from “waking up the sleepy prefrontal cortex” to “targeting correlated or anticorrelated circuits that sort of exist” to “disrupting stuck or unfastened networks.” Honestly, I’m often left with more questions than answers. As much as I’d like one clean, mechanistic explanation… it feels like we’re still just scratching the surface.
Good luck explaining that on a Facebook ad.
A huge thank you to Ben Spielberg for this guest post! If you're looking for care in LA, Ben’s clinic, Bespoke, is excellent. If you are interested in care for depression (or OCD) with TMS at Radial, my day job, here is the link…currently physically in NYC and SC, but more soon.
If you liked this article, please share it!
Some clinics may operate differently, and different countries may operate with different standards. For example, it wouldn’t be totally unreasonable to place the TMS coil over the orbitofrontal cortex, the dorsomedial prefrontal cortex, or the right dorsolateral prefrontal cortex when treating depression. However, it is standard in the United States to start by targeting the left dorsolateral prefrontal cortex. Also, devices like Brainsway include broader targets that implicate some other areas in addition to the LDLPFC.






I’ve done two rounds of TMS, and, unfortunately, I don’t think it helped me. I’m curious to know more about what factors might affect efficacy. Do we know which patients do and don’t respond? The only thing I felt was heightened emotion and irritability for a couple hours, which I was told was a good sign, but nothing ever came of it. Do we know whether that effect is actually a correlate with remission? The only paper I’ve found on this particular effect left me skeptical.