Oh my God, this drug. What can I say about Ambien? I don't remember. And that's the point. The only useful thing Ambien did was spawn some of the funniest memes on the Internet. Also, adolescents are getting very, very high.1
the drug's users sometimes sleepwalk into their kitchens, claw through their refrigerators like animals and consume calories ranging into the thousands.
Zolpidem is the generic name for Ambien. It has an FDA label for short-term insomnia. The actual text of what the drug maker went back and forth with the FDA on is highly instructive; …the FDA begins…. “You prefer an indication and use section that does not include a…(next, a screenshot for vibe…)
The initial approval trials for zolpidem for insomnia demonstrated that a brief course of the medication increased the amount of sleep by 44 minutes. Ambien was the foisted upon the world. It was quite the regulatory dance:
Searle's Ambien (zolpidem tartrate) will test the depth of the market niche for a new, non-benzodiazepine sedative/hypnotic, beginning early in 1993. FDA approved the company's NDA (19-908) on Dec. 16 after a 47-month review and a busy autumn of back-and-forth negotiations.
Forty-seven months of back and forth. What could they have been debating?
Subsequent research concluded:
A meta-analysis of a randomized placebo-controlled trial was used to evaluate the effectiveness and safety of Zolpidem in the treatment of insomnia disorder for one month.
In my life, I have never met anyone who said:
“I’d like to sleep better for up to one month. After that time frame, I’m thrilled to discontinue whatever was helping, given a paucity of data to support its safety and efficacy.”
I am a psychiatrist. I have heard almost everything. This “short term insomnia only” language however, is insane beyond anything I have heard in any psychiatric hospital, therapy setting, emergency department, or panicked phone call.
Short-term insomnia isn’t a problem people ardently seek medical attention to address. And suppose you see your doctor after a few nights of insomnia only. In that case, I can say, with some confidence, that is another problem—and I am looking at you, cortico-striato-thalimo-cortical brain circuitry, and reassurance seeking as a compulsion.
Pointlessly Reassuring Research Supports Ambien’s Safety in People With Simple Problems
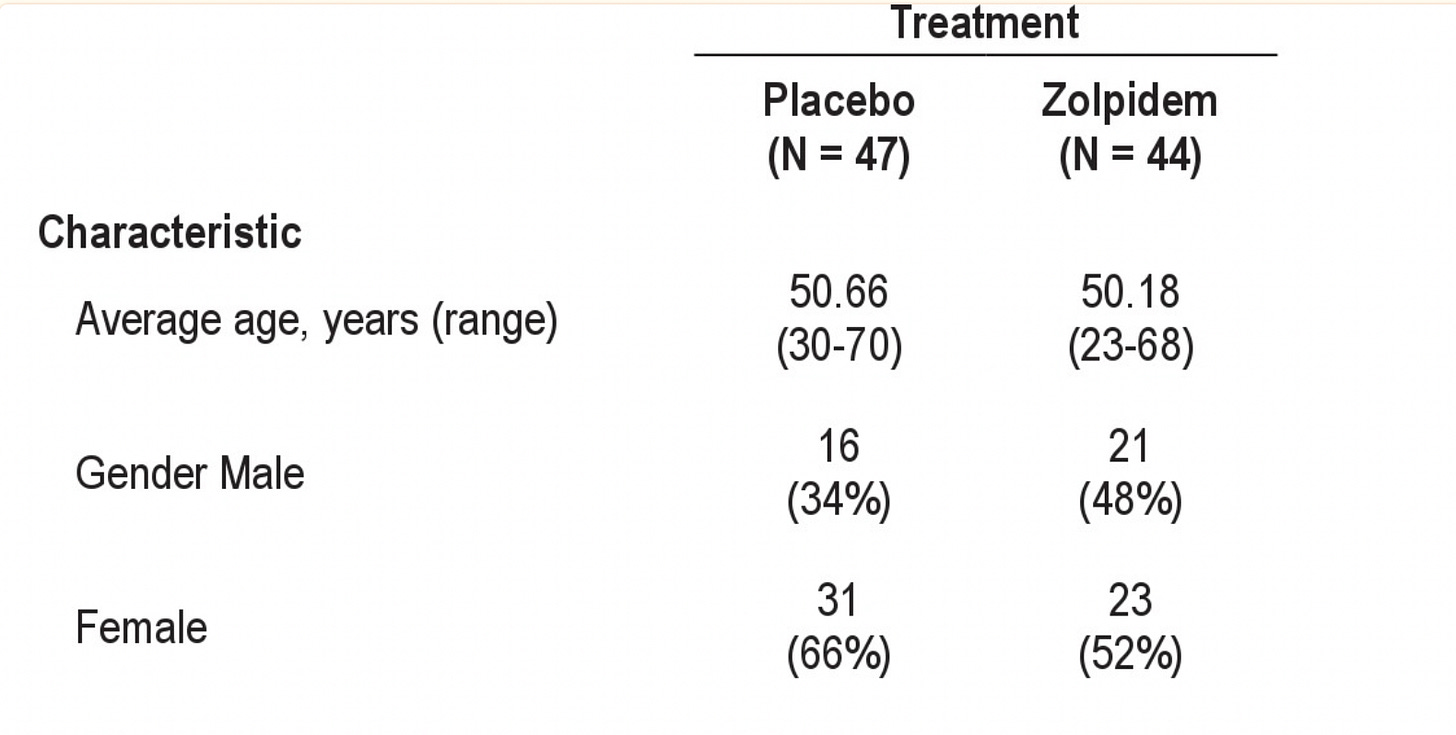
A Subsequent RCT looked at the longer-term use of zolpidem and assured us that randomization was successful (in Table 1—Owen’s favorite table, as my readership is well aware):
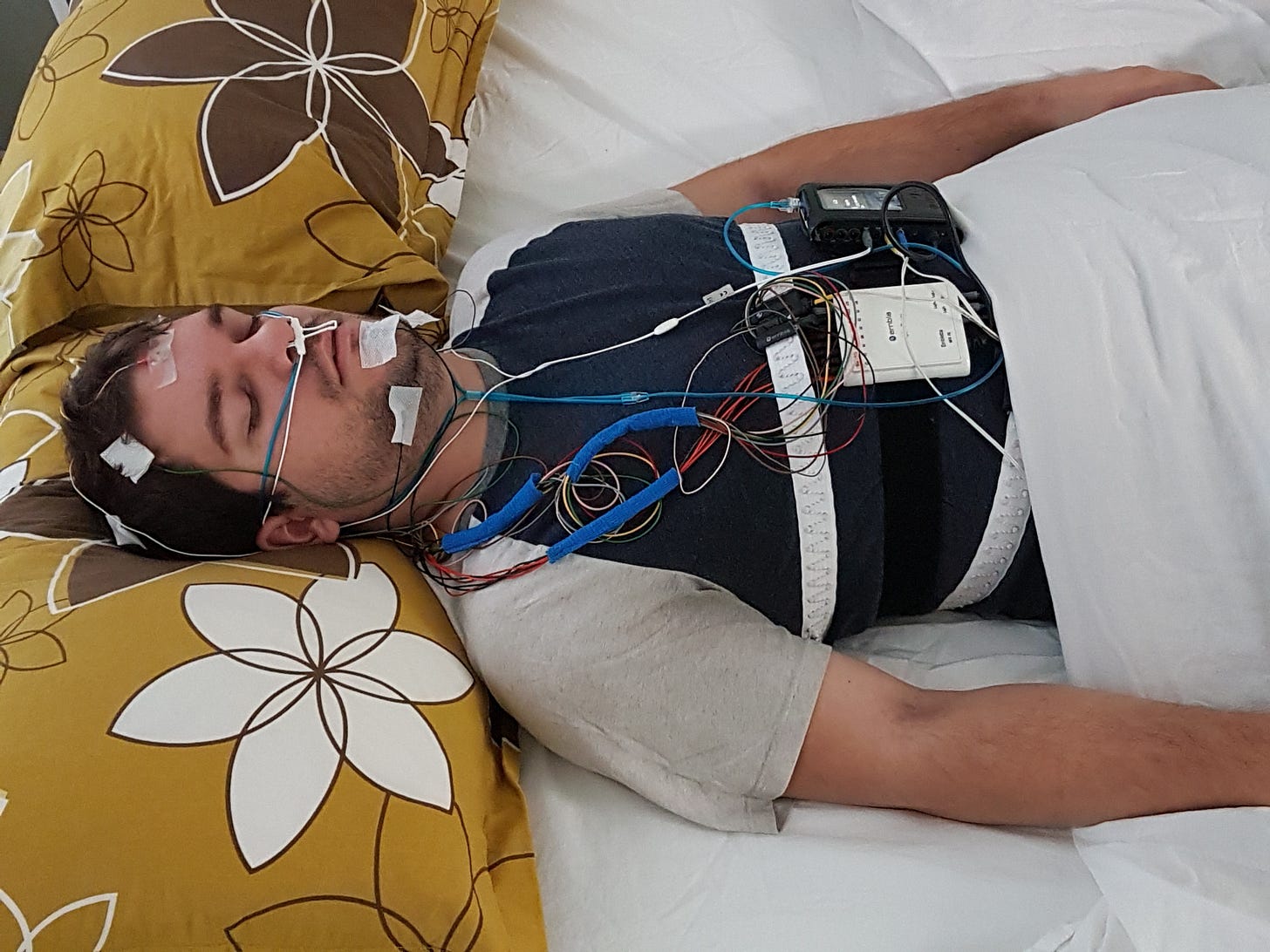
They got a sample of people with chronic insomnia—but no other problems—who were willing to be randomized to placebo or zolpidem, and be measured with pre-post polysomnography and self-report over eight months.
This is what polysomnography looks like:
Subjects had to agree to be screened before enrollment, and this was done for two nights in month one and two months in month 8 of the study. No one had a psychiatric disorder or any other significant medical condition in the study2.
Quick—find your friends who have 11 years of chronic insomnia. Now, subject them to a research-grade assessment to ensure none have any other psychiatric problems. Or save time and ask, “how is your mental health?” and exclude all enthusiastically well individuals from further consideration. Next, offer them eight months of randomized treatment with a medicine that might help them sleep, but only by 40 additional minutes. Also, they have to agree to not drink alcohol for 4 hours before bed for eight months. Now tell them it is for science. Tell me how many “yes” replies you get to that amazing offer.3
The base rate of psychiatric illness is 25% in any given year. In individuals with chronic insomnia, it’s 40%.4. Let’s return to this study; 370 were screened. They ruled out an almost perfect 39% for psychiatric illness, 17% for medical illness, 18% for sleep apnea on screening Polysomnography, and 26% for bailing on bonkers study design—sorry, “other reasons.” 125 people said yes to eight months of study. This excludes 100% of the people in whom, as a psychiatrist, I would be interested. I mean, what could go wrong?
By design, this study excludes anyone I’d ever prescribe anything. This sample feels full of lies. These lies are either on the part of the subjects or the investigators..and maybe not big capital L lies. It might simply be a careful study that misses the point entirely—these individuals don’t require the most detailed further study. Others do.
This is an implausibly perfectly enrolled study in implausibly untroubled individuals. All that having been said—This is a NIDA-funded trial. It is not industry-funded. The authors did it with R01 Research funding from NIH. It could be completely valid in the cohort of people who magically have only chronic insomnia and want to spend eight months in an RCT with no other problems.
What is the problem? Ambien is so safe!
The axe to grind here? These are not the right questions. This study is reassuring about how safe this medicine is for some remarkably resilient people who didn’t have co-occurring depression with 11 years of chronic insomnia. The same group is so agreeable as to consent to participate in that trial, blithely. It doesn’t address the vulnerable individuals, those for whom insomnia is a risk factor for completed suicide, who will be treated with this safe-in-others medicine.
What about those other imperfect insomnia patients? You know, like this author. Yep. I had Ambien prescribed in my 20s—it wasn’t for just one month. With my bipolar disorder, I would have been excluded from the NIH-funded study. You might have been excluded, just by virtue of reading this psychiatrist’s newsletter, too.
What happens in the real world with Ambien?
Tolerance develops rapidly to the sleep-inducing effects of zolpidem. You don't get more sleep after you've taken it for a while. I know studies of perfect samples say otherwise.
People like me, however, do forget that they were staying awake at night because tolerance does not develop for the amnestic effects.
This is the perfect drug….