Depression Can Be OVER: 78% Remission in a New Study!
Hanlon, et. al. (including your author) publish the largest clinical trial on accelerated TMS to date.
Well, thank goodness it’s been a slow news month, and the world, out of sheer boredom, has its eyes on this very newsletter.
Today, this very morning, there is breaking news out of…me. Ok, in fairness, I’m a middle author on the forthcoming publication. It’s actually news from Hanlon et. al. and study sponsor BrainsWay. The study in question is the largest (ever) multicenter, randomized controlled trial (RCT) evaluating accelerated transcranial magnetic stimulation (TMS) for the treatment of depression. The paper, submitted but not uploaded to a pre-print server, so you will all have to WAIT PATIENTLY, is called:
Accelerated Deep TMS for Depression: Results from a Multisite, Randomized Non-Inferiority Trial.
The trial enrolled 104 subjects, which is 3x larger than the SNT trial, and was able to get so large without being shut down because it used a novel design for this type of trial— non-inferiority. 100% of research subjects got an active treatment! Let’s review what we (as investigators) did:
Patients in the accelerated group completed five sessions per day over six treatment days, followed by a brief continuation phase of eight sessions over the subsequent four weeks.
This was compared to once-a-day high-frequency TMS, as already approved by the FDA, and with published results demonstrating 65%+ remission rates in a large post-marketing data set, by Roth et. al.
This non-inferiority design was asking the question, “Is accelerated TMS worse than this other active treatment, which we already know is good?” This study approach was addressed in a recent podcast of mine with my friend and fellow TMS innovation maxi, Jonathan Downar.
The study results were still a surprise…in that they were bonkers good:
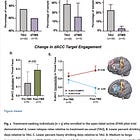
The accelerated Deep TMS group achieved significant improvement that was comparable to the standard Deep TMS group:
HDRS depression scores were reduced by 18.9 and 19.9 points in the accelerated and standard Deep TMS groups, respectively; and
Response and remission rates were 87.8% and 78.0%, respectively, for the accelerated group, compared to response and remission rates of 87.5% and 87.5%, respectively, for the standard group.
78% remission of depression without an fMRI, and with only five treatments (a “half day” compared to the 79% remission rate in the SNT trial is…a good outcome. Now, when people get to read the paper, these were different cohorts, and that isn’t an apples-to-apples comparison—the SNT trial included individuals with the most severe treatment-resistant depression (13 prior unsuccessful medication trials on average). This study would have screened out nearly 100% of people who were included in the SNT trial.
However, in the much larger (n=104 vs n=29), the fact that accelerated TMS wasn’t worse than once-daily treatment—there was no placebo group—is a big deal.
Congrats to all the authors on this publication, and to the study sponsor, Brainsway, which has been making moves, deploying its mountain of cash-on-hand in the press, for getting a complex study done!
Most importantly, I want to thank the study subjects who participated in this trial, because without their bodies and brains, we’d never know depression could be over in so short a time. Research subjects — not all heroes wear capes.
If you want to get the kind of care that gets your depression into remission, Radial (my company) provides it! Now, we are in New York, La Jolla, Myrtle Beach, and more soon!
If you enjoy this newsletter, subscribe and share with your friends!
Prior Articles on the topic include:
And more…subscribe and use the search function!









My concern... From what I've understood, you've shown that a treatment X is just as effective in your average community depressed patient as another treatment Y is in severely depressed patients... Given your average community patient has much less severe illness, and thus remission in four to eight weeks occurs in at least half of your "cured" patients naturalistically, my takeaway would be... Don't recommend this for severe depression!
It also doesn't appear to have any specific efficacy for community depression (given this is easier to treat, if it did, I'd expect a better response rate than with the treatment resistant cohort) - my takeaway is that this treatment appears to be less effective in the community cohort, given half of the improvements would have occurred as part of the natural history of the illness, which is an effect you don't see in the treatment resistant cohort.)
It's not surprising... Community depression is often "shitty life syndrome" misdiagnosed and responds differently to true melancholia (if that was what the treatment resistant cohort had, and not just ongoing crap social situations!)
I am interested in a response, this is a medical student's take.
When these results are released it will be useful when speaking to funders. Keep up the good work!