Insurance Plan Requires More Studies on Parachutes: “Unproven and Experimental”
Prior Authorization, from the Prince of Lies?: “The Passion of the Medical Loss Ratio,” Part 2.
Welcome Back, Dear Readers!
A review of the First Part:
The Medical Loss Ratio (MLR) (a fixed profit margin) means ever increasing healthcare spending.
So, limits on access to mental health care is the profitable strategy for “the payers.”
Last time, I compared the Medical Loss Ratio, unfavorably, to crucifixion. I am edgy! It’s a hot take for a physician-writer, but if you are reading this article, it worked— where is that on your checklist, Dr. Gawande?!

Given the ever increasing size of our major healthcare conglomerates, we can presume they have mastered the MLR math. Also, Amazon bought One Medical last week, and hot takes from the Twitterati blew the Heck up like a cute girl Twitter account making an inane comment:
Now that we are up to speed…follow as the magic unfolds. With incentives for cost to go up, it will happen!

My argument is that since psychiatric illness is a major driver of general medical spend, we have a mental health system working as intended.
The “I’m cutting costs” story is one any health insurance employed human can tell themselves, and enjoy restful sleep at night.1
The payers aren’t—strictly speaking—to blame for perverse incentives (granted they may have done some lobbying). They are generating shareholder value! Expecting them to lower costs, in violation of their fiduciary role to shareholders, however, is a fever dream. It’s not a broken system, it’s an extraordinary machine.
This second article is an examination of a system “built to spill,2” while maintaining plausible deniability. No CMO or Medical Director is required to be Dr. de Sade.
“Unproven”—A 3rd Party Payer Rationale… or an Opening Band at an Emo Show?

“Unproven and Experimental” treatments are excluded from health plans. This can even include FDA-approved treatments (Quoting the FDA website here):
FDA Approval: What it means
FDA approval of a drug means that data on the drug’s effects have been reviewed by CDER, and the drug is determined to provide benefits that outweigh its known and potential risks for the intended population.
Most mental health care is “carved out“ — this means that it is in a separate company silo. No budgets allocated to the care of the brain need to intersect with the budgets allocated to the care of the body- until the quarterly earnings call for the whole company.
Insurance companies seem to love to exclude psychiatric treatments from their coverage. They can’t just do this without any rationale.3. So they come up with a rationale that sounds plausible. They deny care that is “experimental and unproven.”
The FDA, meanwhile, has this concern as an express part of the approval process for all treatments:
Assessment of benefits and risks from clinical data—FDA reviewers evaluate clinical benefit and risk information submitted by the drug maker, taking into account any uncertainties that may result from imperfect or incomplete data. Generally, the agency expects that the drug maker will submit results from two well-designed clinical trials, to be sure that the findings from the first trial are not the result of chance or bias. In certain cases, especially if the disease is rare and multiple trials may not be feasible, convincing evidence from one clinical trial may be enough. Evidence that the drug will benefit the target population should outweigh any risks and uncertainties.
We who wish to have our patients’ care paid for are asked to pretend, like an insurance reviewer who “wants to hear about the case” before the denial, that the extensive efforts of the FDA should be replicated to satisfy the crack science squad carrying the bag for capricious denials?
Prior Authorization, or, Sisyphus Gets A Gig as a Claims Reviewer After Finding His Last Job Too Fulfilling
The trick comes when we start to look at what any definition of “experimental” or “unproven” might be. Although payers are endlessly interested in demonstrating their value proposition:

Payers don’t define “unproven” and “experimental” however. Why should they—with a loose enough definition for those terms, literally everything in medicine can be spun to be experimental and unproven:
Dr. Muir: “the patient has X condition, with a rating scale score in the very severe range. He has previously been treated to remission for X psychiatric condition with Y treatment which is FDA approved. All other treatments have failed. You are now denying this care with Y treatment. [Sup]?”
Dr. Whatshername, Medical Director: “It’s a plan exclusion! We don’t pay for it because it’s unproven and experimental!”
Dr. Muir: “He’s been a plan member for years, and in my prior clinic I was allowed to treat him under the contract we had with you. I know it works for him because your company paid for it. I’m also the co-author of the largest data set of post-marketing results for this FDA approved treatment. Have you reviewed that data?”
Dr Whatshername: “I don’t feel comfortable with this.”
Dr. Muir: “You don’t feel comfortable talking about medical data”?
Dr. Whatshername: “ I feel this isn’t going anywhere. This is disrespectful. It is unproven and experimental.”
Dr. Muir (letting the snark get the best of me): “Please define your understanding of “unproven” for the purposes of this review?”
(The call didn’t last much longer…)4
I imagine Dr. Whatshername and I could agree this call didn’t need to happen. It was Kabuki of the damned. Neither of us were able to be honest. Dr. W was allowed to say “No.” My options were similarly limited. Perhaps I’d lash out in impotent rage? At best, I would have the Pyrrhic victory of making a colleague feel shitty about her life choices.
Remember, in the biomedical sciences, we never prove anything. We can demonstrate likelihood. We can replicate those findings. The FDA kinda has this game on lock. But the very fact that we are using statistics means that every “proven” treatment is fair game for revision.
Even jury instructions understand that there are shades of certainty: reasonable doubt, for example! Demanding doctors ignore generally accepted evidence is a humiliating immolation of thousands of physician hours. But at least the Payer employed bagmen—sorry, reviewers—get paid for the time spent. Those advocating for care do not5
TRUST ME: A Pivotal Fake Study
Trains Running Unhindered Suck for Those who Might have an Encounter: a Sham Controlled Double Blind Study
To be clear, what I’m describing is based on a real study of a treatment for a psychiatric condition, which I have replaced with Getting Hit By a Train. Below is the abstract—a summary of the paper—almost word for word.
Our Experimental Treatment is Brakes.
The question I need you, dear readers, to consider: should this treatment be covered by payers? Or do we consider this unproven and experimental and demand more data?
I present my made up FDA clearance trial! It’s of bFRT (breaks for runaway trains). Our authors of TRUST ME demonstrate, in those those randomly assigned to be hit by a runaway train, the clinical efficacy of bFRT. (Edits are only for grammar after my find/replace:)
Objective:
Getting Hit By A Train (GHbT), it is a chronic and disabling condition that often responds unsatisfactorily to pharmacological and psychological treatments. Converging evidence suggests a dysfunction of “breaks on the runaway train” in GHbT, and a previous feasibility study indicated beneficial effects of breaks for runaway trains (bFRT) targeting the impact at high speeds as a key pathway. The authors examined the therapeutic effect of bFRT in a multicenter double-blind sham-controlled study.
Methods:
At 11 centers, 99 at-risk-for-GHbT patients were randomly allocated to treatment with either bFRT or sham bFRT (the break lines were cut but conductors and subjects were blinded to sham vs. active assignment) and received bFRT or sham treatments for 6 weeks. Clinical response to treatment was determined using the Is Your Skull Fractured Yet Scale (IYSFYS), and the primary efficacy endpoint was the change in score from baseline to post-treatment assessment. Additional measures were response rates (defined as a reduction of ≥30% in IYSFYS score) at the post-treatment assessment and after another month of follow-up.
Results:
Eighty-nine percent of the active treatment group and 96% of the sham treatment group completed the study. The reduction in IYSFYS score among patients who received active bFRT treatment was significantly greater than among patients who received sham treatment (reductions of 6.0 points and 3.3 points, respectively), with response rates of 38.1% and 11.1%, respectively. At the 1-month follow-up, the response rates were 45.2% in the active treatment group and 17.8% in the sham treatment group. Significant differences between the groups were maintained at follow-up.
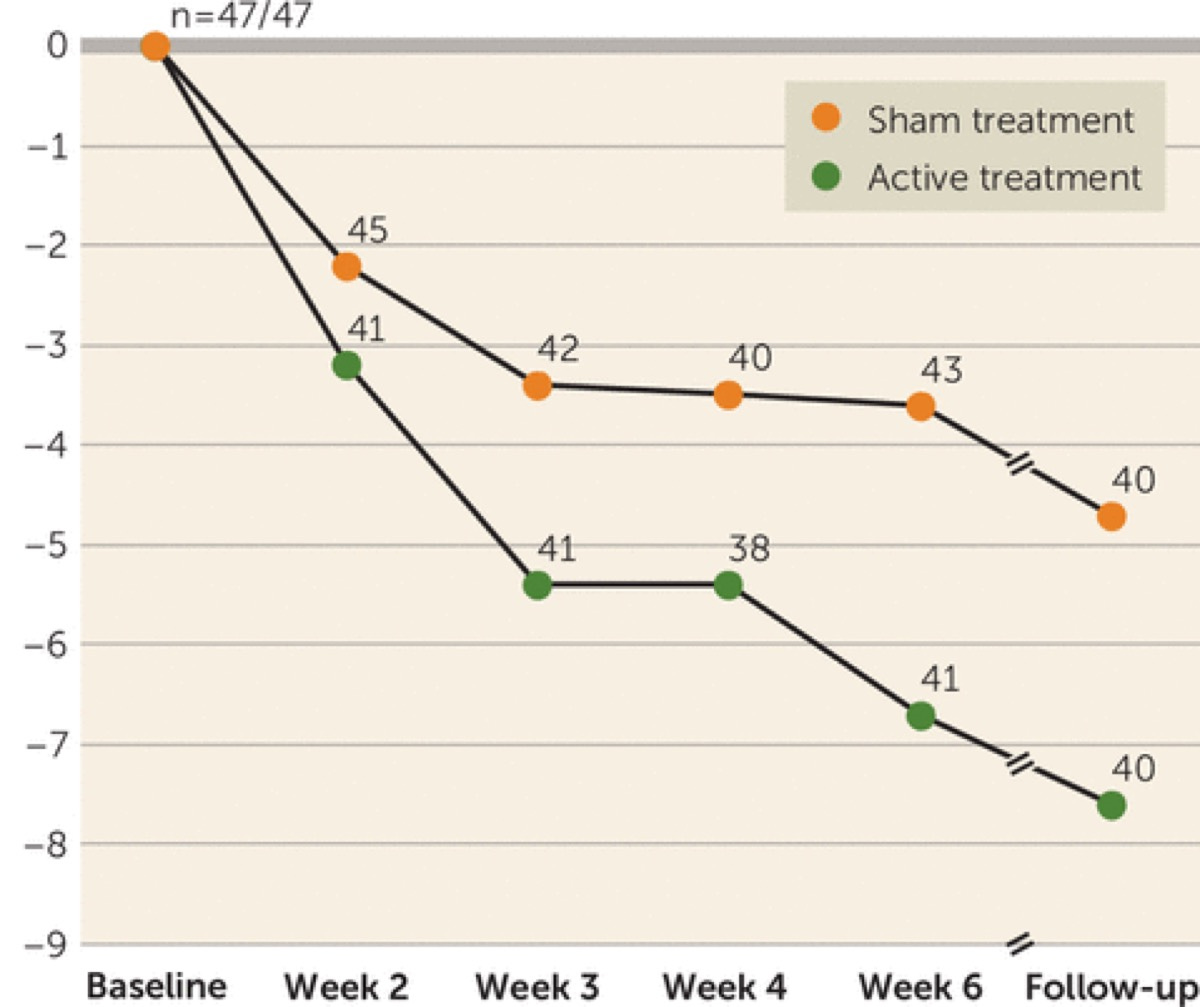
Conclusions:
High-frequency bFRT improved GHbT symptoms and may be considered as a potential intervention for patients who do not respond adequately to pharmacological and psychological interventions.
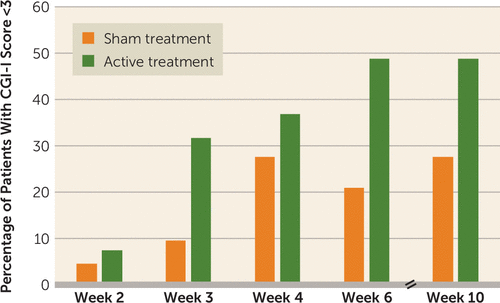
Summary: 27.4% less skulls bashed in after being hit by a train. This was statistically significant! That is a call to action moment:
The FDA considered TRUST ME compelling evidence of the novel treatment, and awarded clearance to the real world version of bFRT for the psychiatric equivalent of GHbT.
The FDA nod was in August of 2018. I will note that the real world treatment this satirical study is based on has the same safety profile as brakes on a train—they are both loud and that is about as bad as the risks get.
The big reveal is that this treatment is for OCD. It’s using deep Transcranial Magnetic Stimulation (TMS) with the H7 coil made by BrainsWay.
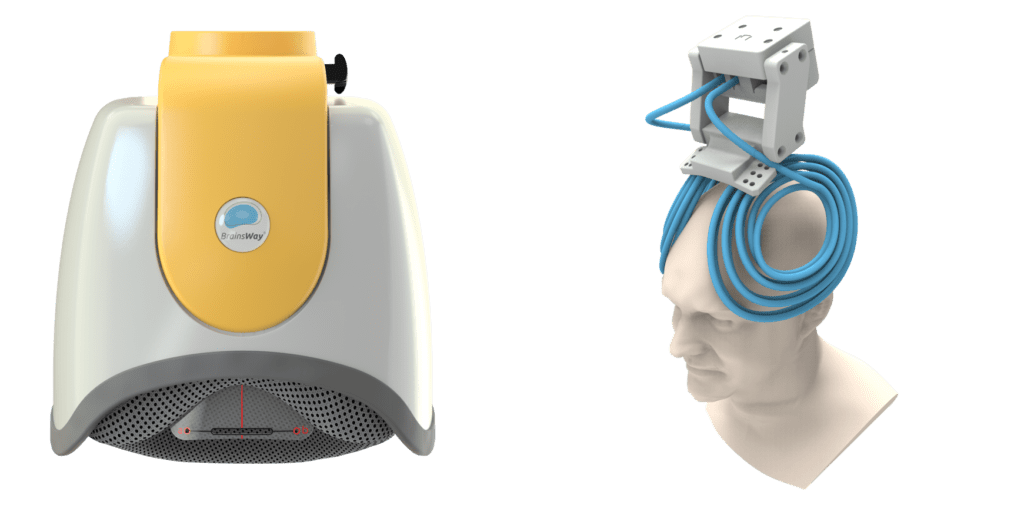
For some reason, it’s absurd to think that we would allow head injuries from trains hitting you. But it’s an acceptable plan exclusion when your own brain is causing the tremendous suffering. Even when the Clinical TMS Society has conveniently and professionally outlined when it should be included as a covered service? United Healthcare, along with most major payers, says, “No thanks, we’re good.”
At a certain point, Institutional Review Boards will stop studies midway if the active treatment is so much better than the sham treatment that they have to call it early.
It is unethical6 to expose human subjects to sham treatment if it answers no meaningful questions7
History has not been kind to the doctors who exposed human subjects to the dangers of controlled studies8 for the purpose of trivial or evil questions. The Nuremberg Trials, anyone? It’s like sham-controlled parachute studies for the falling—not ok. 9
It feels a little bit like Dorothy trying to get out of the land of the Wizard of Oz, on hold with a reviewer:
“There is Someplace Like Home, but We Are Afraid You Don’t Meet Three-Heel-Click Criteria at This Time.”
When it comes to attempts to deal with this fiscal minefield, employers have adopted a strategy, increasingly, of just paying for their own health care themselves, and that’s having more control over their plan design and benefits. This aims to control costs10 while providing better care. This approach is called “self funding,” and employers that use it hit a perfect 100% Medical Loss Ratio by not profiting on the process. It’s still another visit to Golgotha, but crucifixion is no longer mandatory.
My Spirited Dissent…Won’t Matter.
Insisting on non-scientific standards of proof beyond replicated studies with statistically appropriate data sets, and even FDA approval or clearance is inhumane.11
If we had more treatments that lead to remission paid for by payers, it would would mean less depression, PTSD, OCD, and more. But... Less profits. The key to profitability of our largest health care companies is the ability to control everything:
With all these different businesses, it is theoretically possible for one piece of Optum to be reducing a hospital’s cash flow by denying medical claims for United subscribers, while United’s health insurance network managers bargain aggressively to reduce the hospital’s reimbursement rates while yet another piece of Optum runs the billing and collection services for the same hospital and its employed physicians, while yet another piece of Optum competes with the hospital’s physicians and ambulatory services, diverting patients from its ERs and clinics, reducing the hospital’s revenues.
Damned if we do, and damned if we don’t. But the infinite refinement of the ability to control the medical loss ratio is the only rational business strategy for our massive health care conglomerates. And who could blame them? It’s a bit like raging against the barbarism of crucifixes, but only taking specific issue with the asphyxiation. It was, and is, kind of the point.
“The Fall”: That time we promoted Lucifer to Senior Vice President of Value Based Suffering at the newly acquired OneHell subsidiary of Heavenly Health:
If the above sounds like a sinister plot to make sure people stay mentally ill, I wish it was. That would be a Sin, for which we could beg forgiveness. Satan in charge would have left corporate memos, discoverable emails, incriminating text messages- there would be a paper trail. But you don’t get to see all the pieces at any given vantage point in healthcare megacorps. It took this long just to explain it. You need to do your job. Everyone is doing a little piece of work, in their different silos, and the goal individually is not being fired. Claims are going to be administered. Claims are going to be denied. Prior authorization is going to be required. The machinery of prior authorization will be tinkered with. It’s an invisible maze more complicated than whatever the hell Arnold’s Game is using on Westworld. And the suffering is just as gruesome as what the Romans built for systematic crucifixion, but with none of the transparency.
Jesus, Etc.
Recall, Jesus had to be killed because he was a dangerous radical, and a threat to the financial interests the day.
Could I get in trouble for writing this? I mean, what are they gonna do, crucify me?
—O. Scott Muir, M.D.
Confidential to my readers currently in Payer jobs: sorry about any symptoms of emergent insomnia! Have your heard about Lunesta? I’m kidding of course, it has needed some adjustment by the FDA!
In case anyone wants to see Built to Spill, best band out of Idaho, on tour with me…
This is the real-life paraphrased transcript of my “peer-to-peer” with a reviewer at a Payer just this week… and for full transparency I let them know this piece was coming.
Can you imagine a lawyer putting up with this indignity and not billing for the time?
Tuskegee Study of Untreated Syphilis for example.
So after some serious consideration of the feedback of my editor/wife, I’m taking this David Foster Wallace level digression and putting it where he would argue it belongs: in a footnote. I clearly have a real axe to grind about the denials for Transcranial Magnetic Stimulation, a treatment with a fabulously low marginal cost, but in fee-for-service land is routinely denied, including when I needed it:

Payers Regularly:
require prior authorization for the most effective treatments
deny objective reality when it comes to what medical necessity criteria should be, and I will reference this Optimus Prime Health guidelines:
With the assertion “Theta burst stimulation is currently unproven”…in 2021, but when I did everyone the solid of googling that for them:
It seems as if their guidelines conflict with, you know, 2019 facts? Now we all agree that an FDA breakthrough status is not an FDA clearance, but in the case of theta burst stimulation and it’s accelerated variant, it’s really hard to understand any actual logic behind its denial. It’s like denying 80 mg of Prozac versus 40 mg of Prozac. Which to be fair they also do with some regularity…but so the tirade makes some sense, keep in mind that I’m both a biased TMS doctor, and the thing people are requesting coverage of doesn’t have to cost anymore than the thing they’re already paying for. It’s the same device, FDA cleared, just safer and more tolerable in the case of Theta Burst, and that same Theta Burst more often in a day instead of over several weeks.
That guideline is from 2021. One could wonder if they had contented themselves with 2016 reviews? Clearly someone must have avoided reviewing the Stanford “accelerated” results, also released in 2021. And the similar approach previously reviewed in 2019 in a meta-analysis by Corker and colleagues. Or perhaps they missed the promising open label results published by Eleanor Cole, et. Al. In 2020. Or even work previewing the future published by my group in 2019 and again in 2020 or as far back as case series evidence in 2018. Ellie Cole PhD (first author on Stanford lab Accelerated TMS paper) describes this really well:
Don’t worry, if that is too cutting edge, they also require endless notes and regularly deny or just don’t pay for FDA cleared rTMS or dTMS either.
Despite epic publication between 2009-2018:
The FDA approval of rTMS in 2008 that was for treatment resistant depression with 1 failed medication trial. Payers still routinely require 2-4 failed med trials. And 1-2 failed therapy trial. In the current episode of depression.
This is not because these restrictions are based on broad evidence or decades of research. Why, then, are these approval criteria? Oh, it was the average number treatment trials in the 2008 NeuroStar approval trial.
The requirements also demand—get ready for the sacrifice— the the first born child of the prescribing psychiatrist. I at least have two. They are twins after all.The recent payer requirement for the prescribing psychiatrist to be physically on site for the delivery of a treatment by technicians robs first born kids of their parents on the regular. It’s a little bit like requiring Ronald McDonald himself to be present at every single McDonald’s at which a hamburger is sold. Except it’s hard to argue that TMS is riskier than french fries production or consumption.
Maybe it has something to do with the fact that the remission rate for TMS treatment of Depression is Between 30 and 50% for once daily TMS treatment and up to 80% for accelerated treatment. This is compared to 10% remission rates of small molecule oral medicine treatment in the same TRD cohort.
The call for more proof when it’s not required is beyond disingenuous. At its extreme, it makes us all into Dr. Joseph Mengele.
In keeping with my attempt to emulate the David Foster Wallace Approved approach of relentlessly footnoting: The gold standard in terms of savings—and for these employer groups, savings is money in their pocket—is the Validation Institute. The Validation Institute exists to verify hard dollar savings. It turns out it’s really really really easy to claim you’re saving someone money. In the Byzantine maze of healthcare, the actual proof of this requires data scientists and actuaries to take a really detailed look at if this is the case. And according to the wonderful people at the Validation Institute – and I mean they are wonderful, we have talked, I have hired them, and I will again given half the chance – Major Depressive Disorder as a co-occurring diagnosis in the medical chart of an individual leads to a 44% increase in cost over non-depressed peers.
This “depressed costs more” finding has been replicated repeatedly:
In mean costs, depressed elderly patients averaged an increase of 763 US dollars to 979 US dollars in ambulatory costs and 1045 US dollars to 1700 US dollars in ambulatory and inpatient costs.
Now I’m not going to dive into the deep end of “correlation is not causation”—which is trivial, true, and misses the important point that statistics has gotten more advanced since you were introduced to it in college 20 years ago. The math behind determining causation, as deftly explained for general audiences in “The Book of Why” by Judea Pearl, has advanced quite a bit since most of us were deep in the mathematical weeds.
The very short summary of the above massive body work is that advanced statistical methods can determine the arrow of causation from a statistical assessment. “Correlation cannot prove causation”, but sophisticated statistical analysis has moved well past this basic axiom to be able to know with very high degrees of certainty what actually does cause what.
So given depression, as just one illness, will drive cost, one could wonder if our system was one cynically intended to to prevent it’s effective treatment? Perhaps while continuing to mindlessly chant “mental health is so important”? You know, just like in the Wizard of Oz, the minions of the wicked witch chanting away…
I can still grimly remember the voicemail left by a reviewer declining to cover clozapine, the only medication with an FDA indication for suicidal patients with schizophrenia, on the voicemail of another doctor in my practice…for a patient who had completed suicide two days previously while waiting for this medication to be approved.










