Hold Me Closer, Robitussin: is Auvelity (dextromethorphan/bupropion) something new in depression treatment?
Basic science, deftly applied, combined old compounds to bring us the most promising oral antidepressant in 60 years.
This article is one of my all-time most successful pieces. Tonight, I'm focusing on making dinner. It's been that kind of day. And I have many other articles in process, none got over the line. So, this is a rerun. I'm proud to say I still think this article is funny and informative. However, I think it's notable how quickly AI art wore out it's welcome. Humans love novelty, but when something rapidly becomes too easy, so it's not new anymore, because you've seen it everywhere, it feels stale. A lot of things that AI generates feel stale, which is why I hand write this article with the help of dictation software on the daily. Today, you're getting an oldie but a goodie. Thanks for reading The Frontier Psychiatrists, and please consider sharing the work with your friends.

This has been a nostalgia heavy week. Not only did I get to eat at my favorite burrito restaurant from my days at Amherst College, but I got to think about Robo-tripping again. First, a moment of full disclosure:
Bueno Y Sano has the best burritos.
Sautéed spinach and garlic. The best burrito. Hands-down. It’s something about that combination—noted even on the menu, written in chalk— this remarkably simple combination of spinach and garlic as one especiale burrito. So special, in fact, that 20 years later, this is still a go-to for me!
You probably thought I was going to disclose that I did some Robo-tripping in college? You would be incorrect. I did not. This is not to say Robo-tripping wasn’t an activity of choice amongst my classmates. To quote the poster advertising one particularly lively party, let’s “Bust The ‘Tuss.” Shame that never became a song.
At this point, for all of my health executive readership who, presumably didn’t hang out with degenerates in alt-rock bands to get to the c-suite, I will define terms: Robo-tripping is what young people call it when they ingest a lot of dextromethorphan (brand-name: Robitussin) and get really, really high.
This used to be a bargain basement way to get wasted. It was pretty dangerous, mostly because if you accidentally took the common combination of Robitussin and guaifenesin, you get sick, or die. The Tylenol that was sometimes included can be deadly, in overdose, too.
Bespoke burritos and college era chicanery aside, dextromethorphan made a big comeback in the pharmaceutical trade news this week! The FDA approved a new treatment for depression. It’s named Auvelity1. It’s a rapid-acting treatment for depression. It turns out to be a combination of two drugs that have been around for a hell of a long time. One of them is bupropion, which we’ve known as Wellbutrin forevah. The other, to the surprise of many, is our old friend dextromethorphan, the cough suppressant!
Can this be some exciting new thing when it’s really two old things?
Well, here’s where it gets really exciting for me from a nostalgia perspective. I spent a significant amount of time in my life as an MCAT teacher for Kaplan. Hepatic (that’s “in the liver”) metabolism didactics are the kind of walk down memory lane that most people only get rarely. Like when they run into their Midsummer Night’s Dream co-star from freshman year at a 20th High School reunion and realize they have so much more in common than they ever knew! There is an opportunity waiting—right here—with an expectant audience of the several dozen people who find this very specific column entertaining! For our narrator, O. Scott Muir, this is basically the one time he gets to walk into any (virtual) room and feel like The Fonz. Or, maybe, Julius Caesar, triumphantly crossing the Rubicon. Finally, at at long last, O. Scott gets to be cool, for the first time since his 1995 turn as Puck.
With My Deepest Condolences to Everyone Who Endured Rap/Metal
Dextromethorphan is a compound we’ve had around for a while. And it was something, and is something, kids right now are using to get high. This article is not about getting high. It’s about a new medicine. A Very Different use case! Others have explained this before in ways I have found boring and uninspired.
So this new drug, it works on the NMDA glutamate receptor. I know it sounds a lot like MDMA. It’s one letter different. But it’s an important letter, and it’s really different.
This is not the first rabbit-acting antidepressant chemical that the pharmaceutical research world has brought to market.
That sentence was supposed to say rapid acting. But rabbit acting is way funnier. It’s the kind of thing that is funny when you are loopy.
Ketamine: Kids Cooler than Me Used to Have Fun at Parties to Which I Was Not Invited.

How genuinely uncool I was2 may seem like a joke. It’s not. I was really not cool. I have never used any drugs recreationally in my life. I never got invited to those sort of parties. OK maybe now I get invited to some of those sort of parties, but it’s sponsored by Pharma and I have to disclose the value of all the snacks made available to me.
Let’s talk about snacks. It lets me explain receptors without having to be completely obtuse. Doritos. They taste really good. They taste a lot. It’s an extremely strong taste because chemical engineers have done an amazing job of making the powder all over those damn chips extremely potent. What does potent Dorito flavor mean in a scientific context? It means it fits just right on your tongue. Extreme Flavor!
Your tongue has taste buds. It has a variety of different kinds of taste buds. Some taste buds are like Lego blocks, and others are like Duplo blocks. Lego blocks click into Lego blocks. Duplo blocks click into Duplo blocks. So flavors from food fit into the taste buds on your tongue, and the better the fit, the more intense the taste. This is a sweeping generalization, but it’s good enough for one to get the picture.
The neurons in your brain work a heck of a lot like your taste buds. But they have two kinds of receptors, generally. One type of receptor is hooked up to equipment that makes things happen pretty quickly. Another type of receptor turns a crank inside the cell that can make a bunch of other things happen, but axiomatically, not as quickly.
Anything that’s going to lead to neurons firing more right now has to happen pretty quickly. The brain has some “fire now”neurotransmitters, which we haven’t heard a lot about until recently. Glutamate is the primary excitatory — that means neurons fire more — neurotransmitter in the brain.
All of the oral antidepressants you’re used to hearing about, and then getting so disappointed by when they don’t work particularly well for a lot of people, worked on things other than glutamate. Glutamate is like rocket fuel, in that you don’t really want to just dump that on the fire, because it’s gonna lead to lots of sparks. So the slow and steady approach—drugs that took weeks and months to work thanks to downstream changes and down regulations of this and that, that’s what we used till now. Prozac. Zoloft. Wellbutrin. Remeron. It’s like a walk down memory lane, for heavily marketed drugs with associated mugs and pens for doctors offices. Those drugs didn’t do things now, more or less, because they didn’t interact with the glutamate system directly. Glutamate makes neurons fire now. I hope we’re clear on that. Without getting too much in the weeds, one of those types of glutamate receptors has the following long name:
N-methyl-D-aspartate receptor.
NMDA. See? It’s not too hard! Just making something complicated to say shorter—this is 98% of progress in science btw. Acronyms.
The way glutamate makes cells fire now, as opposed to later, is that glutamate and the receptors to which it binds—the taste buds of your neurons!—have one specific function. There are a lot of ways of regulating that function, but it basically just opens a gate.
Charged ions, in this case calcium, can flow through that open gate, and we have the magic of depolarization, which is the science term for changing the voltage inside of a cell fast enough for all the other channels—which are based on the voltage difference inside versus outside of the cell—all fly open at once.
When this happens, a neuron fires, and that process is called an action potential. I have an entire other article on that stuff, and I’m not gonna bore you with it now.
Something Fast Happens.
Nobody wants to take a drug and wait for two weeks to get high. Not when they’re trying to party in college they don’t. That would take planning. And college kids, they are terrible at planning in advance!
But my college peers at a certain New England liberal arts college did have a pretty good sense of what worked quickly. That’s why drugs like those that bind to NMDA receptors are so popular among college kids: Brain cells firing now equals excitement. Too much of that can equal bad things, but just enough of it can mean alterations of consciousness, euphoria, and other effects that happen promptly.
Ketamine works on the NMDA receptor (among other mechanisms). Dextromethorphan works on the NMDA receptor.
The first rapid acting anti-depressant drug approved by the FDA is esketamine, a.k.a. Spravato3, and it’s an intranasal formulation of the anesthetic agent ketamine. It’s an NMDA receptor drug. “Ketamine: it’s a now thing.”
Both of these medications actually antagonize binding sites the NMDA receptor. So it’s regulating a now thing. The important thing to understand is that it has rapid effects because these are ion (calcium aka Ca2+!) channels.
And lo and behold, our new dextromethorphan/bupropion combo pill has an FDA label that indicates it take it can work on depression—as quickly as one week. I grant that this isn’t “now”—but it’s a hell of a lot faster and we are used to seeing with drugs that don’t work this way! Is nothing good enough for you people? This is why I didn’t want to go to your dumb parties anyway, Delta Kappa Epsilon!
Oh, We’re Halfway There, Woah-Oh, Dosin’ on a Prayer!
We are half way there because dextromethorphan is half of the pill and “faster” is only half of the story.
To be clear—you can’t just drink robotussin and have it be an antidepressant.
Bupropion is not part of this pill because it’s an anti-depressant. Or not mostly. If you wanted it to be an antidepressant, you could have just have taken Wellbutrin in the first place. Probably at a higher dose than what is in the new pill! There is a special interaction that happens when you take these two compounds together. That interaction is the magic that turns short acting getting high on cough syrup and turns it into a rapid-acting antidepressant that works faster than every other pill. I’m not gonna make a quick as a bunny joke. I have a sense restraint. I will inhibit myself. Just like…bupropion does.
It’s alchemy! More concretely, it’s like a mashup song. Mash-up songs take ingredients from other songs, but the resultant outcome of that mixing is more than the sum of its parts. Girl Talk, for example, is an unbelievable mashup artist who makes the dance floor go wild. I think it’s fairly safe to say that the level of danceability of any given Girl Talk mashup is significantly higher than whatever rap song and rock anthem went into the musical blender in the first place.
There is a meaningful interaction between the cut of pieces of music that make something completely new out of two old things. You could also mix red and blue paint together, and you’ll get purple. We all agree purple is not the same as red and is not the same as blue.
In The Liver, it’s Where the Magic Happens
Our livers are constantly creating chemical mash-ups, and just as some combinations lead to hotter dance tracks than others, so too does the combo of these drugs allow something totally new as an outcome. So bupropion? It doesn’t just work in the brain. That is the hot trick here. It’s works in the liver. And what it does in the liver is slow down how dextromethorphan is metabolized so that a very little bit of it—way below college kid raucous party amounts—can stay in your body way longer before being itself broken down in the liver. It’s so slow that it has entirely different uses unlocked by this process. The bupropion allows for a dextromethorphan remix—what was a relatively high dose needed in a short lived compound becomes a long lasting compound capable of antidepressant effects that work fast, are relatively safe, last awhile, and don’t mean the people taking it are constantly wasted. It’s basically a completely different drug when it’s allowed, by it’s collab with bupropion, to stick around. Just as the breakdown of a pop song can make it really pop, so two can be inhibition of the metabolic breakdown of one drug change the utility of another.
Perhaps they should have brought this drug to market like a pop star doing a collaboration with a rapper: Dextromethorphan (feat. Bupropion): “Girl, Don’t Be Sad No More.”
It Takes Two to Make a Thing Go Right
DJ Easy Rock and Rob Bass missed their calling —why be mere hip hop artists when they could have been in direct-to-consumer pharmaceutical marketing? In order to create Auvelity, the first rapid-acting oral antidepressant, it took two medications interacting in a way that hadn’t been done before. It took two. This combination is as different as purple is from blue and red. It’s as different as pop songs that feature T-Pain + Autotune, and pop songs that do not:
“It took two” to make inhibiting a liver enzyme go right. It’s something different. And that is welcome news to people with severe depression who have had to wait forever, or at the very best had to come in for expensive and time consuming procedural treatment like ECT and TMS as the options have been limited. Pharma companies seem to take perverse pleasure when their ads predictably say “ask your doctor about another option for depression.” But in this case it’s actually true, and faster acting on the time scale of a week and not months. It’s an option to have depression be better fast. Like a-singular-week. Not 6-8 weeks-plural. Not “16 weeks to have full effect.” This option didn’t exist in oral medications before this medicine mash-up came together.
It Takes Two to Make it Out of Sight!
In summary: the combination of dextromethorphan and bupropion creates a rapid-acting antidepressant. This happens through the alchemy of bupropion making a small dose of dextromethorphan —working at NMDA receptor so it’s speedy— last so much longer than it could have otherwise4 that it does something new!
Alchemy never turned lead into gold, but biochemistry is turning old medicine combinations into new hope. I haven’t been this excited for a mash up since I heard about Elton John and Britney Spears collaborating on Tiny Dancer just last week. Everything old is new again, but the combinations can be better than we remembered.
By the way, I was so excited when I read this paper I immediately took a photo of the results to text to my psychiatrist wife.
Some things are just better together.
—O. Scott Muir, M.D.
I’m not paid to say anything about this drug and have never taken money from the maker of this drug I’ve never even had lunch on them. This isn’t a virtue per se, just no conflict to disclose.
I will agree the use of the past tense here is a combination of hopeful and aggressive.
Hi, Janssen! Full Disclosure: I’m a contracted Key Opinion Leader Advisory Committee member with Janssen on the Spravato product thus my limited things to say in this column.
Altering the ratio of dextromethorphan to dextrophan, the first order hepatic metabolite after CYP450 2D6 metabolic conversion in the body, from what would be usually higher dextrophan levels. This is a “trick” I’ve used regularly with fluvoxamine and clomipramine as well, except that is 3A4/5 inhibition. See, that drug metabolism stuff from doctor school really can be useful!







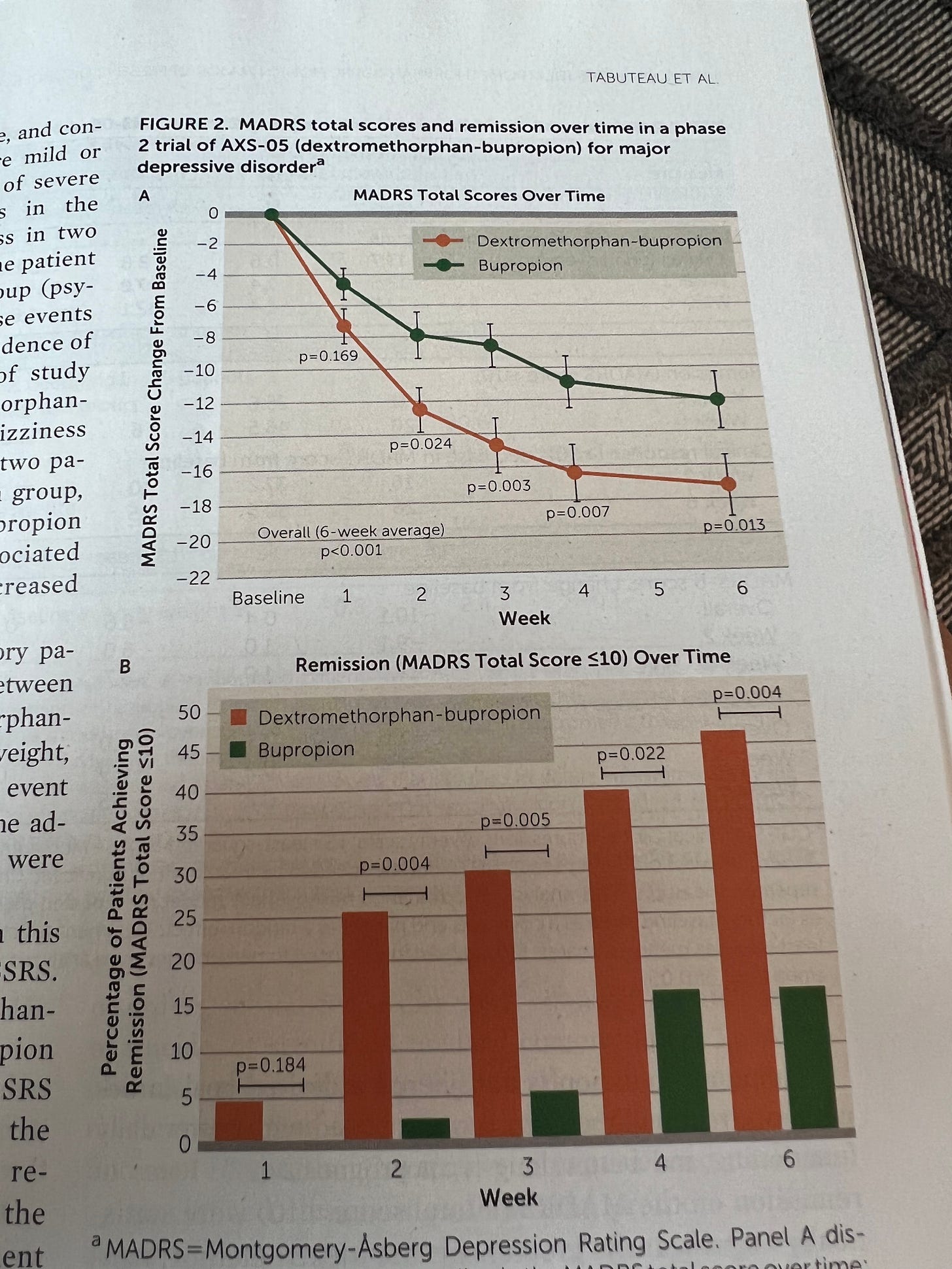
From what I can see, squinting at the table, there is a 2 point improvement on the depression scale at 1 week - are you really so sure that is clinically meaningful? I'd appreciate some guidance on accepted norms around the MDRS scale and clinical meaningfulness. I also note that it only increased to 5 point improvement by the end of the study - again, where does that sit? What point difference can you make on that scale with an improvement in somatic symptoms etc but no mood improvement?
I also note that it seems like most patients still were depressed at the end of the Auvelity study - and this is your yee-dee-dah celebration success?!!
Plus the buproprion only group which seems to be the comparison group had a response rate of only 15%, which is really low - I'm used to response rates for depression treatment more like 40%, so I'm wondering what's going on here??? Make the comparison group look really bad so that you look good in relief? It just raises questions... If the comparison group had the typical inert placebo response rate of 40%, the 45% response rate from Auvelity wouldn't look like much and it's certainly lower than the 60% response rate we were told was the standard ballpark response for ADs
These are genuine questions, I'm not just being a pain-in-the-arse medical student ;)
Cut my life into pieces…