Can rTMS be Used As A Maintenance Treatment in Depression?
The short answer is yes. Thanks, MAINT-R trial!
For years and years, poor souls like your author have provided repetitive transcranial magnetic stimulation (rTMS) treatment for depression (and its cousin, dTMS treatment).
Welcome to The Frontier Psychiatrists! It’s a newsletter, with a psychiatrist serving as the editor-in-chief.
Our patients who were treated with TMS achieved some of the best outcomes in medicine. Just the other day, BrainsWay top-lined a trial (in which your author was an investigator) that demonstrated 78% remission rates…and up!…in treatment-resistant depression. With that kind of ability to relieve severe depression, it would be nice if insurance would cover its use to keep depression in remission. Except, of course, not. Medical necessity policies were laden with my least favorite language… “experimental and unproven” every time the term “Maintenance” was uttered.
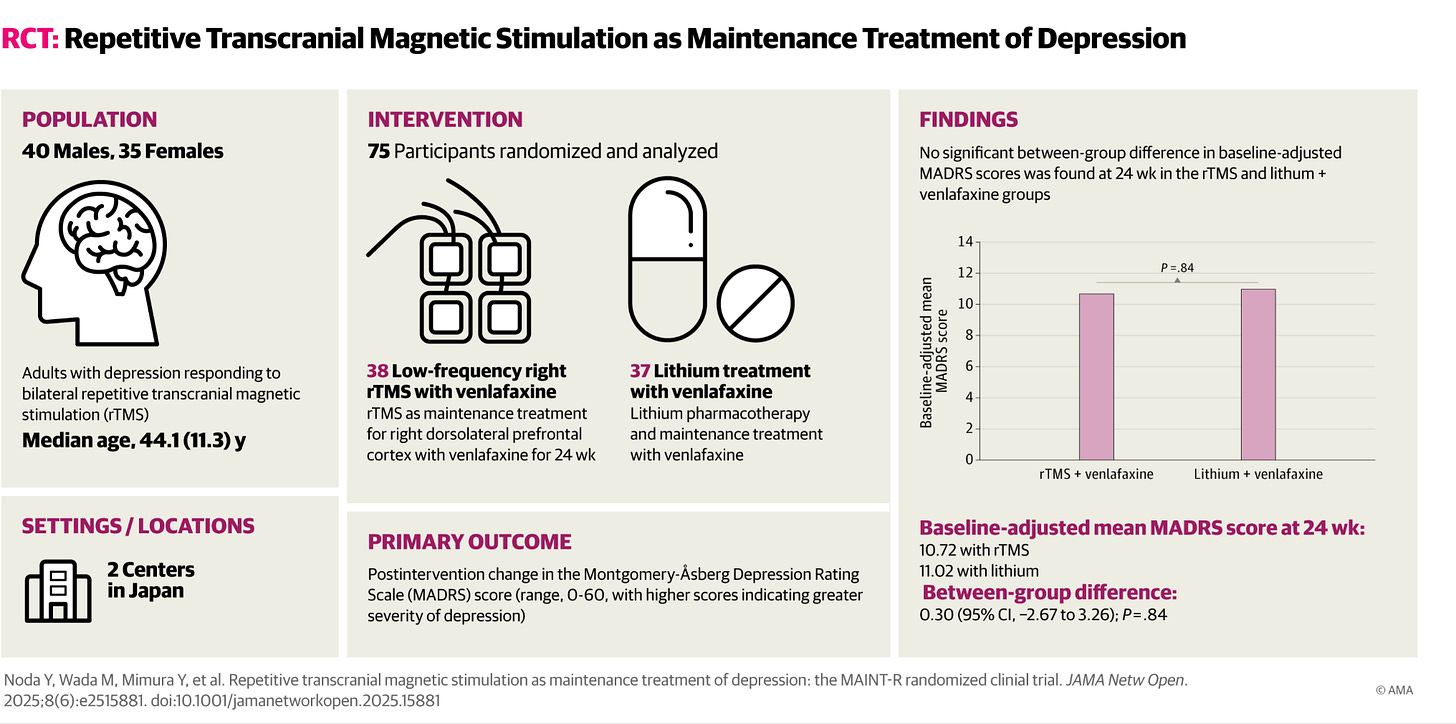
Except…in this case, our payor colleagues? They weren’t wrong. It wasn’t “proven” that continuing the use of TMS at some interval would prevent relapse of depression. It was unproven and would require an experiment to get that proof for us all. However, the ethical design of such a study is a sticky wicket. Do we compare TMS to a placebo? That seems unethical in the context of severe depression. Sometimes, you need an active control. This study enrolled individuals who had achieved remission with repetitive transcranial magnetic stimulation (rTMS) treatment. Then it randomized them to a once-weekly low frequency regimen versus an already proven depression relapse prevention agent—the classic mood disorder agent, lithium.
Repetitive Transcranial Magnetic Stimulation as Maintenance Treatment of Depression: The MAINT-R Randomized Clinical Trial
The TMS treatment was “low dose,” relatively speaking—15 minutes of 1 Hz TMS to the right front part of the brain (The “rDLPFC” for brain nerds1). Usually, in acute treatment, we deliver 2000 pulses over 33 minutes. It’s “TMS light.” Would it still work as well as lithium in preventing relapse to depression?
Noda, et. al.2, working in Japan, where they love robots and TMS, used Kaplan-Meyer survival analysis to follow their 75 subjects, randomized to either 1 Hz or low-dose lithium (after extensive adjustment of the dose to achieve blood levels of 0.4 to 0.6 mEq/L). For more on Lithium level notation, see our recent paper (along with
and first author ).3As a brief aside, this is a pretty fair comparison—it’s lower lithium dosing than generally used in bipolar disorder4, and lower dose TMS than used in acute treatment, in the least aversive form. It’s 15 minutes out of one’s week, plus travel time. These are both realistic “low-dose” approaches, which make sense in a maintenance treatment.
The good news? These approaches both helped prevent relapse equally:
There was, however, a difference in adverse events:
Although there was no evident between-group difference, the number of adverse events was numerically higher in the lithium group than in the rTMS group (16 vs 3; odds ratio, 7.10 [95% CI, 1.84-27.49]; P = .005). Specifically, the lithium group had higher incidences of various adverse events, including depression (n = 3), tremor (n = 3), headache (n = 3), dizziness (n = 2), dysesthesia (n = 1), insomnia (n = 1), decreased libido (n = 1), pancreatitis (n = 1), and hypertension (n = 1).
Lithium had more than TMS.
TMS—it’s as effective as a standard of care approach to preventing relapse to depression…but it’s also safer. It’s no longer “Experimental and Uproven.”
Peer Reviewers working diligently at major payors? Sharpen your denial skills; even more excellent data on TMS is coming your way.
If you are interested in treatment with something other than oral medicines for your depression, my company, Radial, is accepting patients across 18 states by telemedicine and with physical locations in…
Brooklyn, NY
Midtown Manhattan, NY
Myrtle Beach, SC
La Jolla, CA
…and more locations soon!
Fine, the MNI coordinates were as follows: Montreal Neurological Institute coordinates: x = −38, y = 26, z = 44). The stimulus intensity was set at 120% of the resting motor threshold, with the coil placed in the anterior-posterior direction at a 45° angle to the midline. rTMS was delivered using the MagPro R30 TMS stimulator equipped with a B70 fluid-cooled coil (MagVenture Inc).
Noda Y, Wada M, Mimura Y, et al. Repetitive Transcranial Magnetic Stimulation as Maintenance Treatment of Depression: The MAINT-R Randomized Clinical Trial. JAMA Netw Open. 2025;8(6):e2515881. doi:10.1001/jamanetworkopen.2025.15881
Mendelsohn, A., Aftab, A., & Muir, O. (2025). A Proposal for the (Re-) Introduction of Amdisen Standardized Lithium Levels. Bipolar disorders.
Nierenberg AA, Friedman ES, Bowden CL, Sylvia LG, Thase ME, Ketter T, Ostacher MJ, Leon AC, Reilly-Harrington N, Iosifescu DV, Pencina M, Severe JB, Calabrese JR. Lithium treatment moderate-dose use study (LiTMUS) for bipolar disorder: a randomized comparative effectiveness trial of optimized personalized treatment with and without lithium. Am J Psychiatry. 2013 Jan;170(1):102-10. doi: 10.1176/appi.ajp.2012.12060751. PMID: 23288387.





VERY EXPENSIVE! This is pie in the sky until either doctors lower the price or insurance covers treatment . That’s reality.
Fascinating. Question from someone who doesn’t know that much- is this study conclusive enough that we can expect insurance companies to change coverage and doctors to change standard of care, or is the timeline notably longer before any of that begins to change? (perhaps lots more studies are needed, for example)