31% Of People With OCD Could Be In Remission
... if they were given access to an FDA-approved treatment.
I have previously published articles on transcranial magnetic stimulation (TMS) and obsessive compulsive disorder. Obsessive compulsive disorder (OCD) is common, disabling, 10 times more lethal than depression in terms of completed suicide risk, and responds poorly to traditional treatment for 2% of the population.
Definitions that matter:
Response: 50% reduction in symptoms (may still be very sick)
Remission: Symptoms no longer present or so mild they don’t qualify as “disordered.”
Remission is better than response. Remission is what we want from illnesses. Response is what we settle for. But when we talk about evidence-based treatments, we need to ask the question about what the evidence demonstrated the treatment was capable of doing to help. Was remission the outcome? Or was it just response? Now onto the rest of the argument, reviewing recent data about my favorite OCD treatment.
The H7 Coil is a medical device cleared by the FDA that looks like a dopey helmet. It’s manufactured by BrainsWay.
In the journal Brain Stimulation, results from real world clinical care (in which your author is a coauthor) was evaluated by reliable raters using the Gold Standard assessment tool for OCD, the Yale-Brown Obsessive Compulsive Scale (Y-BOCS). The results:
145 patients from 10 sites met the inclusion criteria.
46 out of those have remitted, representing a 31.7% remission rate.
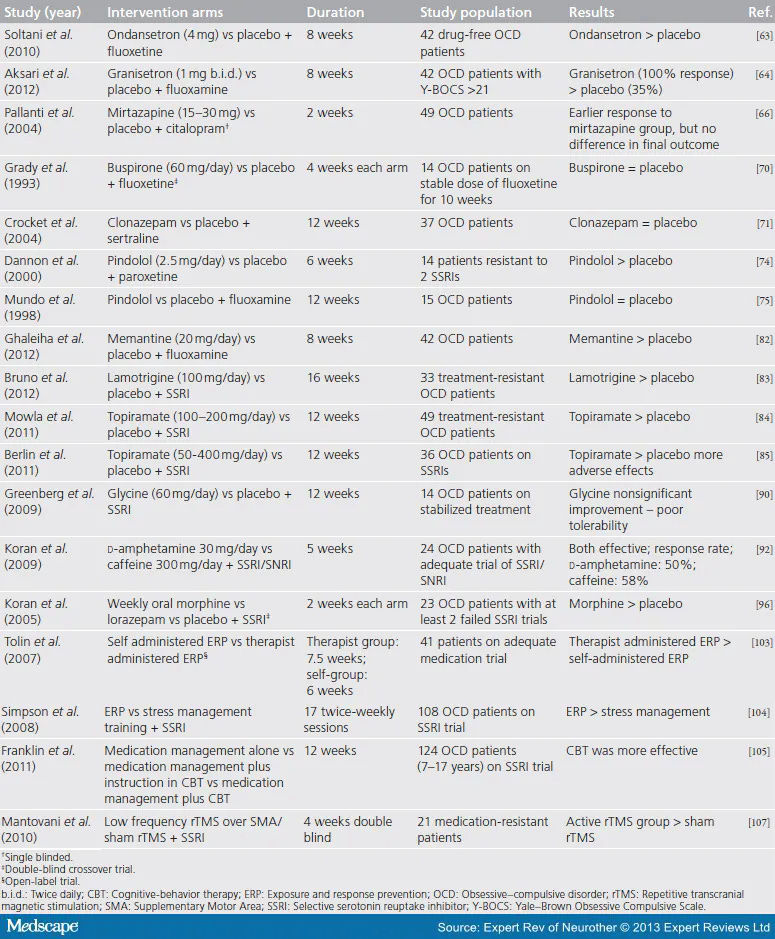
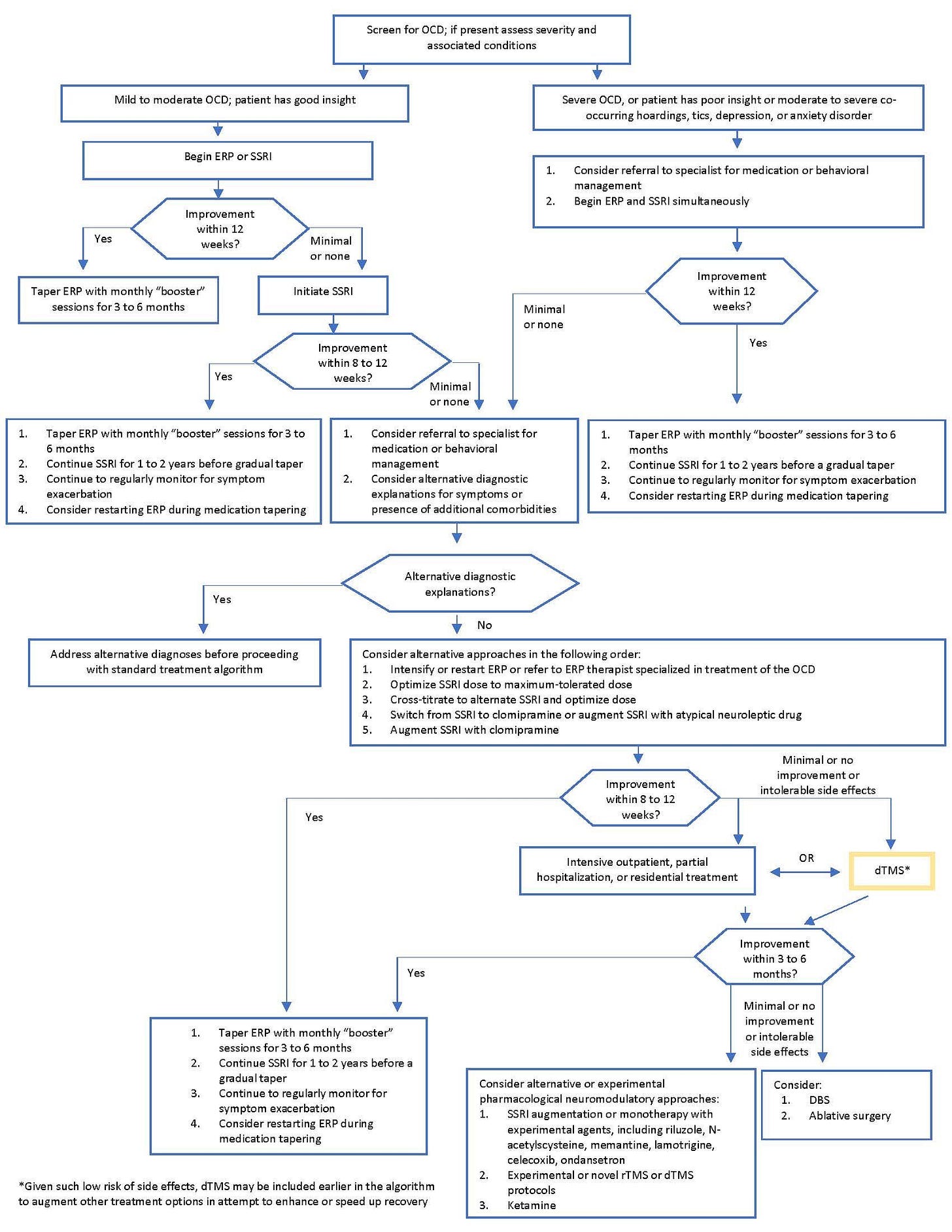
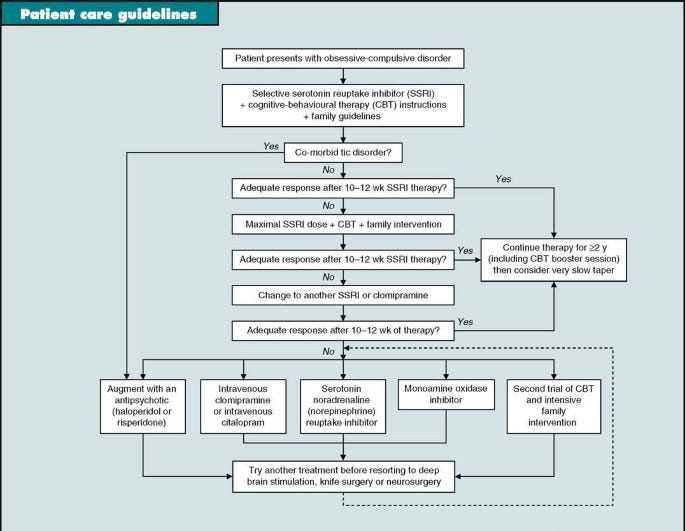
Historically, OCD that isn’t responding well to first line treatment has not responded well to anything else (as many options were evaluated):
The algorithms for “what to do” used to look like this:
Or maybe this:
Or this:
But I’d argue that “guidance” all should now be replaced with this1 when requesting prior authorization from payers who still define this as “experimental and unproven”:
Unlike the treatments that got people a little bit better, for a third of people treated with dTMS, they can have remission of symptoms from OCD. Oncologists don’t treat cancer with the only imagined goal of getting it a “little bit better.” They like remission. Surgeons proudly announce “clean margins” when they resect tumors.
Mental health professionals can start treating OCD with real hope now. A third of people becoming asymptomatic after treatment is a hell of a lot better than no people becoming asymptomatic. And that’s the standard we have been putting up with for years. “Great news, we got it all” isn’t just for oncology surgeons anymore.
That is it. That is the polemic.
—O. Scott Muir, M.D.
ChatGPT was asked for a little bit of help with prior authorization, and it generated this letter to United Healthcare:
Dear United Healthcare,
I am writing to request that you cover deep TMS on a Brainsway device for one of my patients who has been diagnosed with severe OCD that has not responded to medications and therapy. As you may know, TMS has been shown to be an effective treatment option for OCD and has been granted FDA clearance for this indication.
Several studies within the past four years have demonstrated the effectiveness of TMS in treating OCD. For example, a study published in the Journal of Clinical Psychiatry in 2018 found that TMS was associated with significant reductions in OCD symptoms and was well-tolerated by patients. Another study, published in the American Journal of Psychiatry in 2019, found that TMS was effective in reducing OCD symptoms and improving patients' quality of life.
In addition to the scientific evidence supporting the use of TMS for OCD, it is also important to note that the FDA has cleared the use of TMS for the treatment of OCD. This clearance was granted based on the results of clinical trials that demonstrated the safety and effectiveness of TMS in treating OCD.
I strongly believe that TMS on a Brainsway device is an appropriate treatment option for my patient and would greatly improve their quality of life. I respectfully request that you consider covering this treatment for my patient.
Thank you for considering my request.
Sincerely,
Dr. Carlene MacMillan
(Please substitute your name here unless the person reading this happens to be my wife, Carlene MacMillan, in which the above, unaltered, will suffice.)