MDMA: Now Everyone Is Interested in Drug Development!
Lessons From an FDA Process, without commenting on the actual submission in question
As a huge nerd, I feel very seen. Finally, more people than just me care about what the FDA says about a compound. The class of drugs in question are psychedelic medicines, and even the NY Times is all over the FDA process for MDMA-AT.
It’s like Christmas if you are really into supply chains; this is the day everyone becomes interested in global logistics. It is a time for nerds like me to have our expertise shine for one very brief moment.
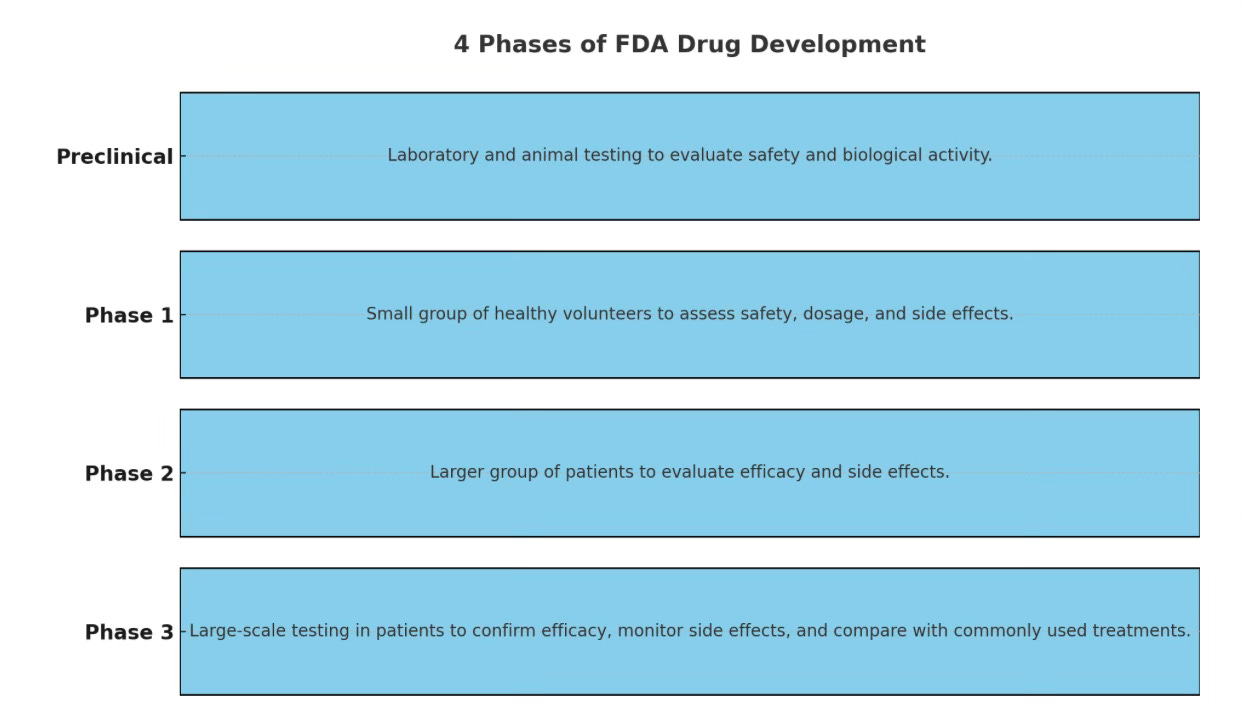
This newsletter has delved deep into FDA filings before…today, a brief overview of the FDA’s therapeutic approval process.
Drugs are submitted to the FDA for approval, with a specific label for the drug's intended use (for example, to treat a disease in a population). This label is crucial because it also limits what marketing is legal! For example, the claim for an imagined chemical that happens to be “water” might be: “for the treatment of mild to moderate dehydration.”
Phase 1
Phase one trials include human health controls to make sure the d…